Publishing
Electronic Nicotine Delivery Systems (ENDS), such as e-cigarettes, are a relatively recent development, so their long-term effects compared to other tobacco products are not yet clearly known.
Because of this, the FDA and Center of Tobacco Products (CTP) developed a new submission standard for Premarket Tobacco Applications (PMTA): The Electronic Tobacco Technical Document (eTTD). These standards can be found in United States Code Title 21, Section 387j under the Federal Food, Drugs, and Cosmetics Act.
Tobacco Publishing
Delphinus was one of the first publishing groups to create eTTDs based on federal regulations, that have since been accepted in all eTTDs submitted by Delphinus and clients using our templates. To request eTTD template information or check Delphinus’s availability to guide your submission, please contact us.
Get In Touch
Consultation/Publishing
In addition to these templates, Delphinus provides our previous experience with eCTD publishing to guide your PMTAs and eTTDs for submission to FDA through consultation and/or publishing services for these submissions.
- Delphinus can aid clients with project management, such as designing the submission layout and timeline, as well as review documents for proper editing.
- Regarding publishing, Delphinus has a 100% success rate with submissions of PMTAs to CTP. Our services include designing an eTTD structure, remediation of documents, generation of a Table of Contents, and submission, pending client review.
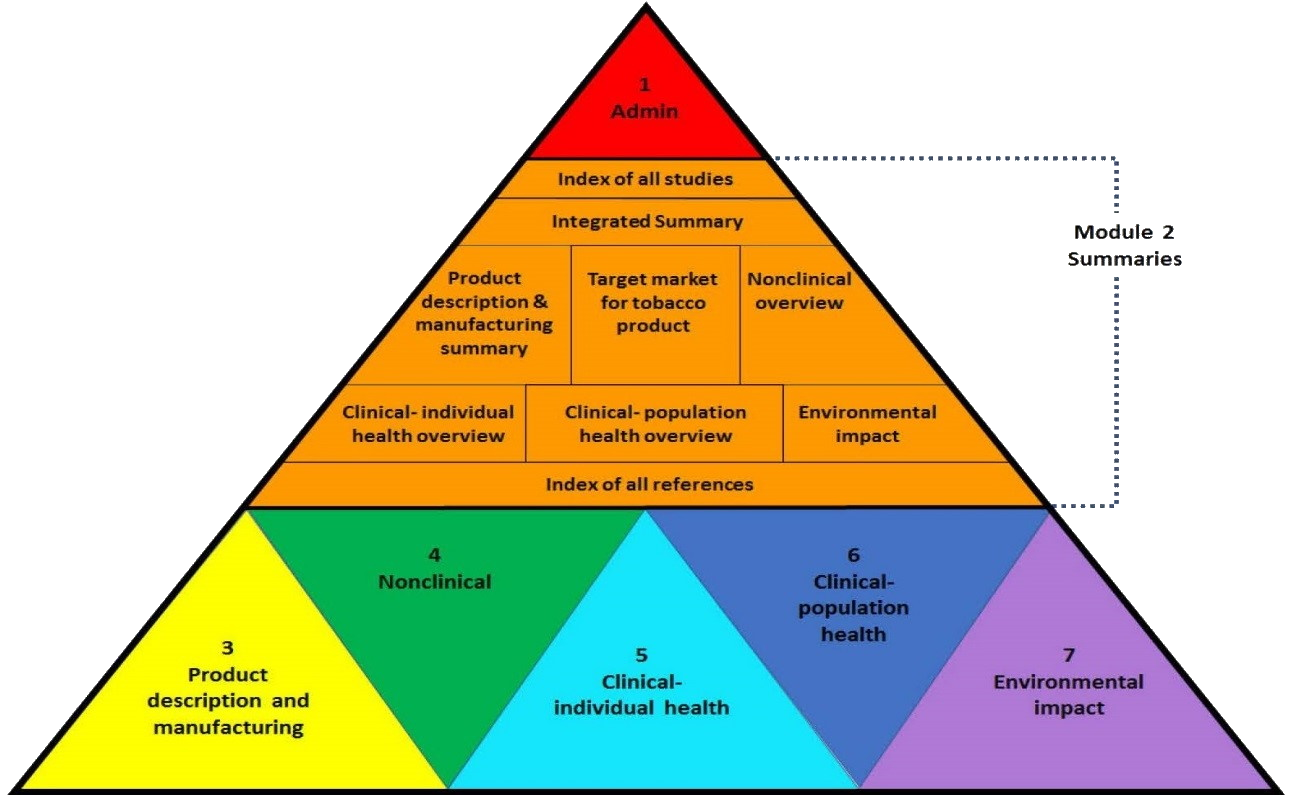
eTTD Structure from CTP
Templates
Delphinus Consulting was the first, and thus far only, to create and market eTTD templates that comply with the new standards listed above.
- Many clients have used our templates to successfully submit their PMTAs to the CTP, allowing them to continue marketing their products.
With the results of our research and expertise, we are proud to offer a collection of 57 Microsoft Word-based templates to assist in the creation of your PMTA.
- Each template contains an FDA acceptable structure, descriptive text, and outlines to help guide your submission, with an example shown below.

Sample eTTD Template
To request eTTD template information or check Delphinus’s availability to guide your submission, please contact us.
