Consulting
Delphinus eTTD Consulting:
Our Means for Your ENDS
Delphinus Consulting personnel have over 40 combined years of experience working with the FDA and CTP as a consultant and submission publisher. The Consulting Services we offer include, but are not limited to:

Get In Touch
Lifecycle Planning and Consultation
Our in-house consultation team can aid clients in the planning and managing of the lifecycle of their PMTAs, from the beginning phases to creating the redacted public records of those finalized PMTAs.
We are continually working with FDA and the Center for Tobacco Products to ensure that the advice we offer falls within their guidelines.
Technical Assessments
Delphinus offers a full technical assessment of existing PMTAs. When purchasing new products, it is important to verify that the PMTAs have been completed accurately and to ensure a valid marketing status.
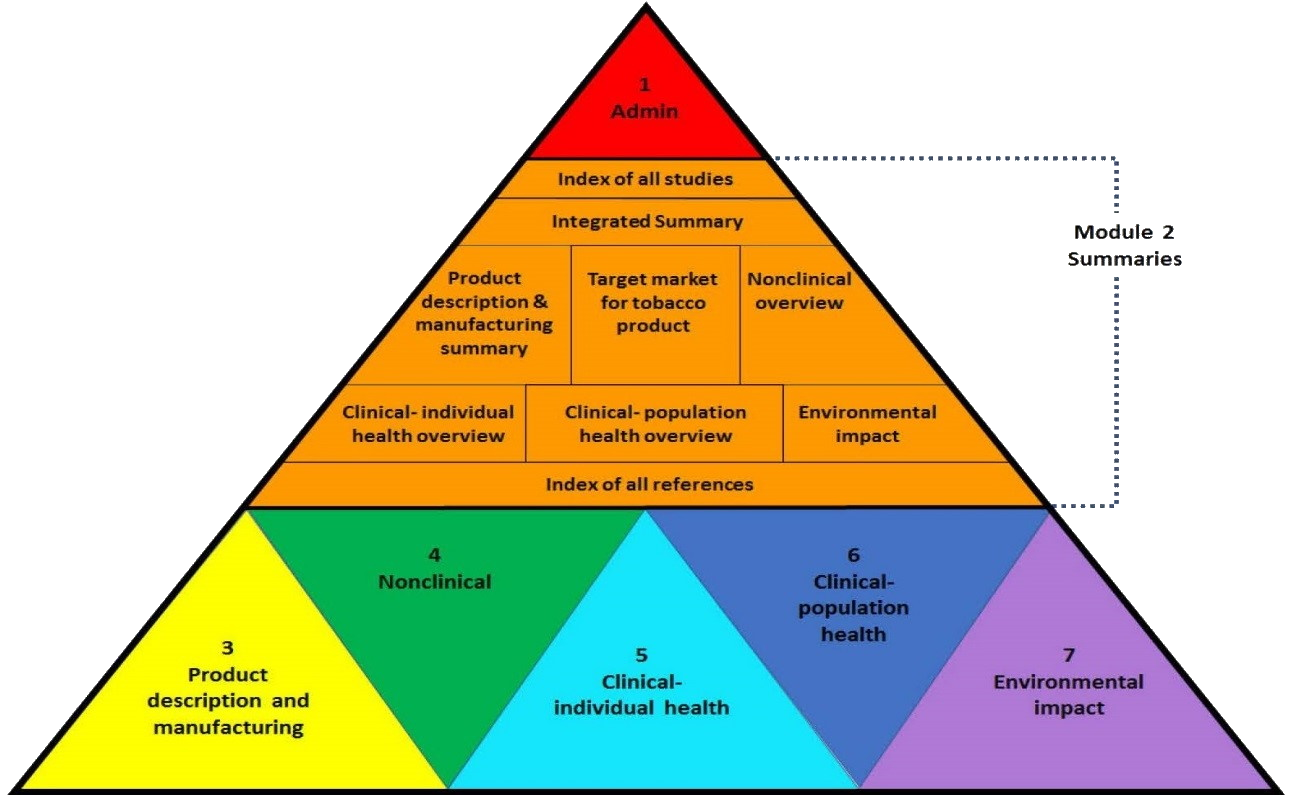
eTTD Structure from CTP
eTTD Templates
Delphinus Consulting has developed a set of templates that will help you understand the complex layout of an eTTD and guide your authors as they write a superb application. These guidelines are an excellent starting point for a new PMTA/TPMF, built with guidance and feedback from the FDA and CTP. For more information, please visit the Support page.
Software Development
Delphinus has software in development to assist the speed and accuracy of PMTA submissions; please check back for new updates.

Sample eTTD Template
To request eTTD template information or check Delphinus’ availability to guide your submission, please contact us.
